Brain’s energy control map offers blueprint for next-gen weight loss therapies
A New Way to Look at Obesity
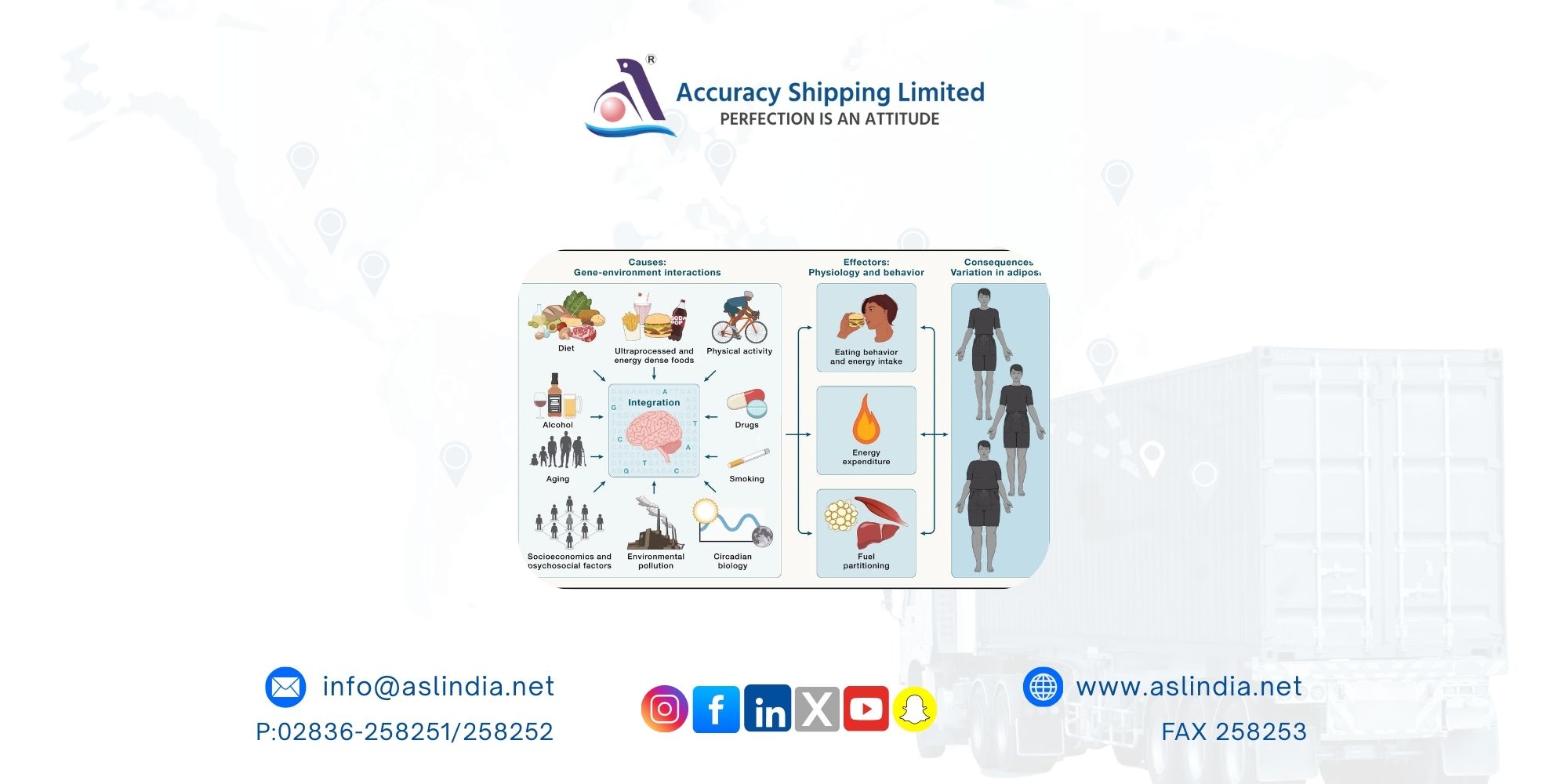
Obesity has evolved from a survival advantage to a global health crisis. Since the 1980s, obesity rates have soared, now affecting around one billion people worldwide. Cardiovascular disease remains the leading cause of obesity-related deaths. Genetics and environment interact in complex ways: some individuals are genetically predisposed to gain weight (“drifty gene” hypothesis), while others resist it. Today’s ultra-processed diets, abundant food cues, and chronic stress amplify these differences. Understanding how the brain, gut, adipose tissue, and liver communicate is essential for creating therapies that achieve long-term, safe weight loss.
How the Brain Balances Energy
The brain integrates slow, long-term adiposity signals with rapid, meal-related cues. Leptin from fat tissue and gut hormones such as GLP-1, GIP, CCK, PYY, secretin, and ghrelin inform the brain about energy status. Neural inputs via vagal and spinal pathways provide quick updates on gut stretch and nutrient content. The liver also contributes with signals like FGF21, IGF-1, and LEAP2. Together, these signals fine-tune digestion, satiety, and metabolism.
The Hypothalamus: The Body’s Energy Accountant
At the center is the arcuate nucleus (ARC) in the hypothalamus, which senses circulating hormones and nutrients. Hunger-promoting AgRP neurons encourage eating, while POMC neurons suppress it by activating MC4R pathways. These circuits adapt to changing energy needs through synaptic plasticity, projecting broadly to influence appetite and energy use.
Hindbrain and Vagal Pathways: Satiety Without Side Effects
The dorsal vagal complex (DVC) helps end meals by processing gut-derived signals. In the nucleus of the solitary tract (NTS), certain neurons can stop hunger without causing discomfort, a key goal for future treatments. In contrast, some area postrema (AP) circuits link appetite suppression to nausea, highlighting why region-specific targeting is important.
Why Palatable Foods Overpower Willpower
The brain’s reward system, involving dopamine pathways from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and prefrontal cortex, assigns extra value to tasty, calorie-dense foods. The lateral hypothalamus connects these reward circuits with hunger systems, ensuring that high-reward foods remain especially tempting. This interplay explains why many people find ultra-processed foods irresistible, even without intense flavors.
From Neural Maps to Modern Medicines
Past obesity drugs targeted monoamines like dopamine, norepinephrine, and serotonin but had limited effect and notable side effects. The rise of peptide-based therapies changed the game. GLP-1 drugs, such as liraglutide, deliver significant weight loss. Dual-acting drugs that target both GLP-1 and GIP receptors can reduce aversive side effects while enhancing appetite control. Amylin receptor agonists, and especially GLP-1/amylin co-agonists like amycretin, have shown dramatic weight reductions up to 24% in trials.
The Road Ahead
We still need to learn which neurons sustain long-term satiety without negative side effects, how stress and diet reshape brain circuits, and how to safely rewire them. Such knowledge will allow patient-specific therapy designs, better tolerability, and extended health benefits.
Conclusion
The brain is the ultimate regulator of appetite, metabolism, and reward. By decoding its complex neuroendocrine map, scientists can design next-generation anti-obesity treatments that maximize satiety, minimize discomfort, and support lasting cardiometabolic health. The future of weight loss may well depend on these brain-based blueprints.